D.A. Petrochenkov, A.M. Natarius
Ammonites of the Ulyanovsk region in jewelry and interior products
Jewelry is constantly in direct contact with a person, which leads to increased requirements for their environmental characteristics. In this regard, we can give an example of charoite - a popular jewelry and crafts stone with a bright purple and lilac color. Charoite aggregates always contain accompanying minerals in various amounts. One of the accessor minerals is ecanite (Ca, Na) 2ThK [(Si, Al) 8O20]nH2O, containing thorium, which creates an increased radioactive background [5]. In individual samples, this background may exceed acceptable standards, which requires constant dosimetric control to reject the material.
To determine the contents of harmful impurities and the radioactive background of stone-colored raw materials, a combined sample of ammonite and septary fragments (simbircite) was prepared. During sampling, emphasis was placed on pyrite-containing fragments, which are most favorable for the concentration of harmful impurities. The sample was crushed and rasterized to the size of the particles necessary for analysis. From a sample weighing 230g, the suspension necessary for the analysis was taken.
According to radiographic analysis (Table 9), the sample consists of calcite (54%) and pyrite (37%). Of the other minerals, there are aragonite of the pearlescent layer of the ammonite shell (7.5%), marcasite (1.5%), as well as traces of quartz and organic matter.
Table 9. Results of radiographic analysis of the combined simbircite sample.
|
Characterization of the sample |
Mineral composition |
Content,% (semi-quantitative) |
|
Ammonite fragments and septarii (simbircite) |
Calcite |
54 |
|
Pyrite |
37 |
|
|
Aragonite |
7,5 |
|
|
Marcasite |
1,5 |
|
|
Quartz |
traces |
|
|
Organic matter |
traces |
According to electron microscopic studies conducted in ammonites (see chap. 3), microswitches of algodanite, pyrolusite, pyrite, quartz, ferrihydrite, apatite, vernadite, spinel, uranium oxide, gold, todorokite, halluasite, monacite, alumosilicates, fine-dispersed formations of graphite, organic matter were found, Among these microswitches are uranium oxide (UO2) and monacite (Ce, La, Y, Th) [PO4] containing radioactive elements.
Uranium oxide (Figure 110) is isolated on the collomorphic surface of the calcite matrix degradation products in the form of a rounded particle about 1 mm in size. Its substance gives a picture corresponding to a cubic face-centered crystal with a spatial group Fm3m and an elementary cell parameter 3.12. In such a group and with such elementary cell parameters, a number of oxides crystallize. Given the form of release, it was assigned to uranium oxide. The microdispersed release of monacite was encountered on a highly altered area of the calcite matrix from the pearlescent layer of the surface of the ammonite shell (Fig. 111).
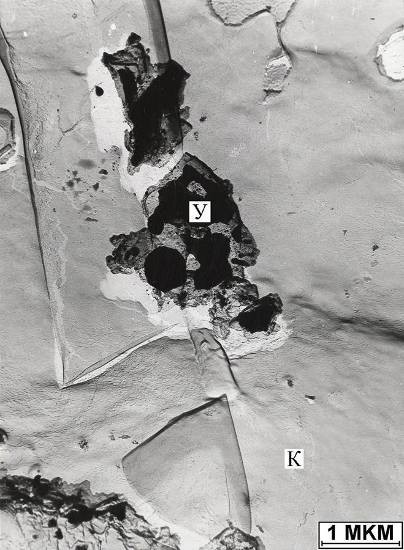
Figure. 110. Oxide microswitching
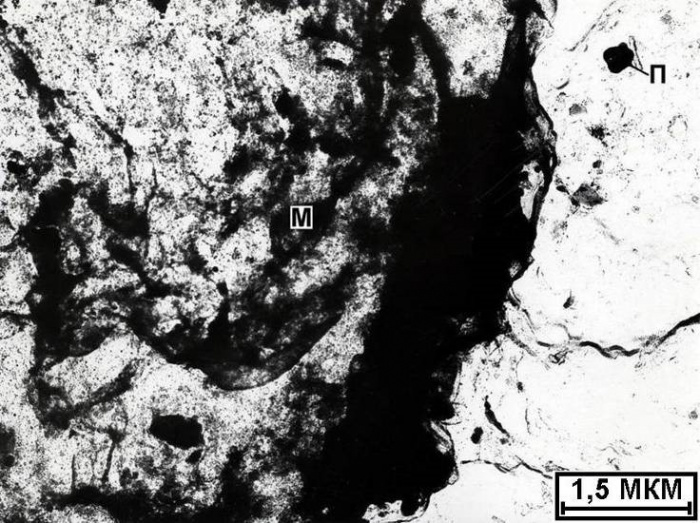
Figure. 111. Microdispersed release of monacite (M) in
uranium (Y) in calcite (K) calcite, P - microswitching of pyrite
The sample was analyzed at the Analytical Certification Testing Center - ASIC VIMS for 73 elements. A number of elements provided for by the methodology have not been identified, many are contained in small quantities not exceeding the sensitivity of the method. From the analyzed elements, the most dangerous for humans are identified, and their content is compared with the established limit standards for drinking water (Table 10).
Table 10. The contents of the most dangerous elements for humans in simbirzite and their permissible contents.
|
Element |
Allowed contents,% SanPiN 2.1.4.1074-01 |
Item Content,% |
|
|
Beryllium |
Be |
0,02 |
0,000037 |
|
Arsenic |
As |
0,05 |
0,00043 |
|
Antimony |
Sb |
0,01 |
< 0,00003 |
|
Mercury |
Hg |
0,0005 |
< 0,00001 |
|
Lead |
Pb |
0,03 |
0,0009 |
|
Cobalt |
Co |
0,1 |
0,00011 |
|
Strontium |
Sr |
7 |
0,08 |
|
Thorium |
Th |
- |
0,000021 |
|
Uranium |
U |
- |
0,00007 |
The table shows that the content of the elements most harmful to humans does not exceed even the permissible standards for drinking water. All elements are in mineral form. The content of thorium and uranium is ten thousandths of a percent, although they exceed the sensitivity of the method. Their contents are apparently associated with the established microswitches of uranium oxide and monacite.
Radiation tests of the sample were carried out in the laboratory of isotopic methods of VIMS analysis (Table 11).
Table 11. Results of simbircite radiation characteristics tests.
|
Sample |
Specific activity, Bq/kg (±∆, absolute measurement error, P = 0.95) |
||||||
|
А∑α |
А∑β |
226Ra |
232Th |
40K |
137Cs |
AEff |
|
|
Simbirtsit |
120±50 |
50±20 |
10,5±3,5 |
1,0±0,6 |
≤ 15 |
< 1 |
12±4 |
The test results show that the measured values of specific total activity of alpha and beta radionuclides (A∑α,A∑β), specific activity of 226Ra, 232Th, 40K, Aeff in the sample do not exceed variations of natural background for non-radioactive rocks and soils of the European part of the Russian Federation and meet the requirements established for:
-1 class of mineral raw materials and products of its industrial processing: Aeff< 740 Bq/kg, SP 2.6.1.798-99, paragraphs 5.2, 5.3 - handling of materials in production conditions without any restrictions.
-1 class of building materials (including sands, limestone, clay): Aeff < 370 Bq/kg, NRB-99, item 5.3.4; GOST 30108-94, Section 3 - can be used in residential and public buildings under construction and reconstruction.
The specific activity of technogenic 137Cs in the sample is significantly lower than the level of typical background values of global precipitation on the earth's surface (5-20 Bq/kg).
The disadvantage of ammonites is the rapid oxidation of pyrite. As a result, the surface of the shell is sometimes covered with gray film, which significantly degrades the appearance of the products. The causes of this phenomenon are serobacteria, as a result of the life of which sulfuric acid is formed. The unstable structure of the pyrite itself plays a negative role, due to the highly elongated shape of the crystals (Figure 112) with numerous pores and cavities on their surface (Figure 113).
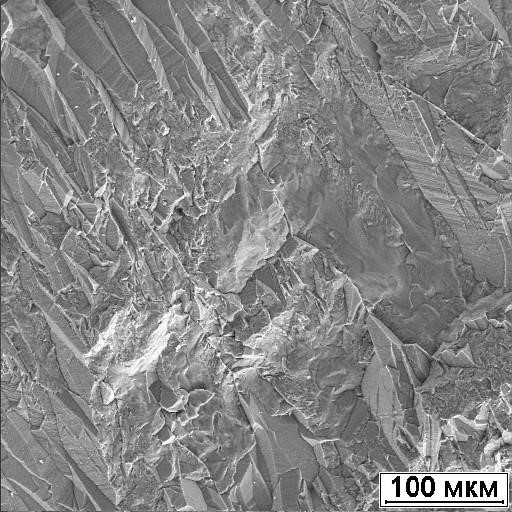
Figure. 112. Elongated crystal shape
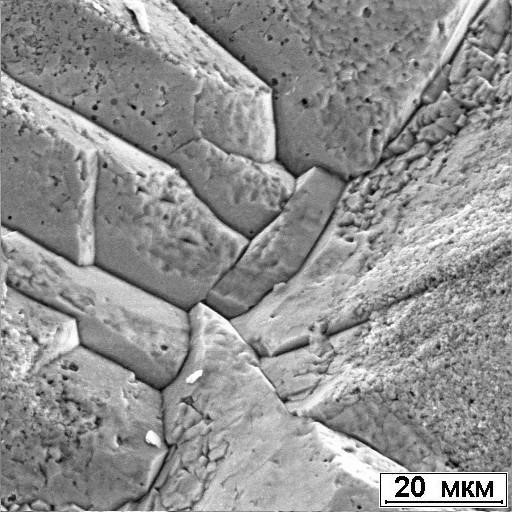
Figure. 113. Porous surface of crystals
pyrite in ammonite
Thus, the studies conducted did not establish an excess of harmful impurities or radiation background, even based on the most stringent requirements. This conclusion is also confirmed by the high demand for ammonite products in the absence of any factors of their negative impact on humans. Moreover, ammonites are also actively used in lithotherapy. For long-term preservation of the appearance of the articles, it is necessary to develop a method for preservation of pyrite.
